
Secrets inside diamonds
Prof Dr Fabian Mohr / Inorganic Chemistry
Photo: Sebastian Jarych
The secrets inside diamonds
Fabian Mohr uses X-rays to determine the molecular structures in crystals
It is the second largest diamond ever discovered. A mining company found the 2492-carat stone comparable in size to an avocado in Botswana, Africa, in 2024 using state-of-the-art X-ray technology. This massive stone is the largest discovery in over 120 years and only smaller than the legendary Cullinan diamond.

The largest diamond ever found, called Cullinan
Photo: public domain
Diamonds are crystalline materials, like sugar, table salt, ice, and snow, but they are much more expensive and, above all, harder to find. Fabian Mohr, professor of inorganic chemistry, knows all about the nature of crystalline structures. “Diamonds were formed billions of years ago in the Earth’s interior from carbon under high temperatures and high pressure,” the chemist says, “and today they can also be produced artificially under high temperatures and high pressure.” Everyone has probably already seen a larger or smaller version of a diamond or even worn one as a ring. The quality and value of the stones depends on the size, transparency and flawlessness of the respective pieces; any inclusions are undesirable, the expert explains.

Seeds of the carob tree
Photo: CC-BY SA 3.0
Carat, the unit of measurement of the carob tree
The value of a gemstone is still measured in carats today. But where does it actually come from? Mohr explains: “The carat is an old unit of weight derived from the seeds of the carob tree. The dried seeds all have approximately the same weight. The word carat is derived from the Greek ‘keratio’ meaning carob tree. If you convert one carat into grams today, it is about 0.2 grams.”
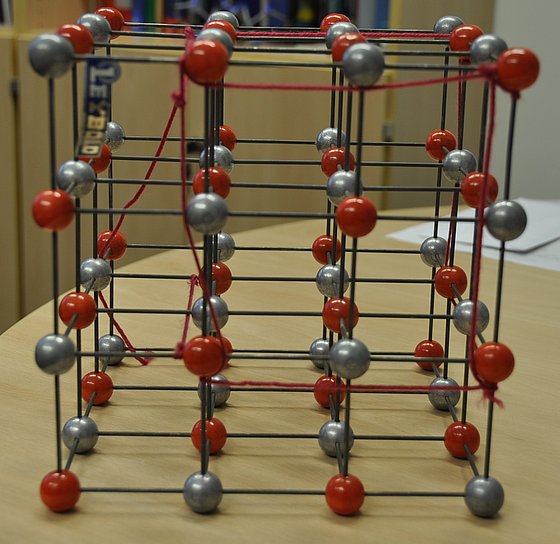
Cubic crystal structure of the diamond
Photo: UniService Third Mission
Diamonds in science
“I have a model that exemplifies what makes a crystal,” the chemist says, showing a three-dimensional cubic structure with a pattern that is periodically repeated by rods and spheres. “And this is repeated again and again, and that’s what distinguishes a crystal from an amorphous material, i.e., a non-crystalline material.”

X-ray diffractometer
Photo: UniService Third Mission
Due to their crystalline structure, diamonds can now be precisely analysed because their atoms are regularly arranged. The analysis of the second largest diamond ever found, mentioned above, was carried out using an X-ray diffractometer, a crystal structure analyser that is also used at the University of Wuppertal for measurement purposes. “We use the device to determine molecular structures,” the expert explains, “i.e., we can use this device to determine the position of the atoms in three-dimensional space from an ideally perfect crystal, based on X-rays, and thus know the composition, the three-dimensional structure of a molecule.” This is particularly important because researchers want to know exactly what a molecule looks like. “In pharmacological research, for example, you want to know what a molecule looks like, because it might need to fit into a specific enzyme.” In medicine, there are various areas of application that are already working successfully with diamonds or other crystalline materials. Dentistry, for example, has been using diamonds in orthodontics, oral surgery, and crown and bridge technology for some time. Although the idea of diamonds as energy transmitters to water is discussed in both esoteric and scientific circles, Mohr explains, who has also seen examples of this at trade fairs, there is as yet no scientific evidence of measurable energy transmission that goes beyond the usual physical properties of diamonds. However, electrical engineering, makes use of the outstanding conductivity of gemstones, as the thermal conductivity of diamonds is exceptionally high. It lies between 1,500 and 2,200 watts per metre Kelvin (W/mK) which means that they significantly outperform other materials such as copper and aluminium and are ideal for cooling high-performance electronics such as semiconductor lasers, high-frequency transistors and LEDs. The exceptional thermal conductivity is also demonstrated by synthetically produced diamonds, so-called CVD diamonds (a CVD diamond - chemical vapour deposition - is a cultivated laboratory diamond. It is chemically, physically, and optically identical to mine diamonds (editor's note). Using crystal structure analysis, Mohr and his team can analyse almost all crystalline materials. New ideas can also be realised with this device. “Everyone is currently talking about alternative ionic materials for lithium batteries, which can also be analysed here. In the research groups currently conducting research, mainly in inorganic chemistry, but also in organic chemistry and macromolecular chemistry, crystal structure analysis is primarily used to elucidate the structures of the molecules being researched ” Mohr concludes.

The 9 largest parts of the Cullinan after the split
Photo: public domain
Incidentally, the largest diamond ever discovered was the so-called Cullinan, found near Pretoria, South Africa, in 1905. The 3106-carat stone was cut into pieces and has been part of the British crown jewels ever since.
Uwe Blass
Prof Dr Fabian Mohr studied chemistry in Melbourne (Australia) where he also obtained his doctorate. After working in Canada, the USA and Spain, he has been Professor of Inorganic Chemistry in the School of Mathematics and Natural Sciences at the University of Wuppertal since 2014.
