
How messenger substances of nerve cells cause electrical signals
Prof. Dr. Julia Bornhorst / Food Chemistry
Photo: Sebastian Jarych
Frog hearts in three-four time
or how messenger substances of the nerve cells cause electrical signals
Food chemist Prof. Dr. Julia Bornhorst on the discovery of the first neurotransmitter in 1921 and current research at Bergische University
Until 1921, it was still believed that only electrical impulses mediated between nerve cells. Exactly 100 years ago, however, the physician Otto Loewi was able to prove the existence of chemical carriers. How did he do it?
Bornhorst: Henry Hallett Dale and Otto Loewi together received the Nobel Prize in Physiology or Medicine in 1936 for their discoveries in the chemical transmission of nerve impulses. In 1921, Loewi was able to discover the chemical transmission of nerve impulses. It is an exciting experiment for which he used frogs. He removed the heart from the frog and placed it in a separate beaker containing a saline solution. Then he stimulated the frog's vagus nerve, one of the longest cranial nerves that we also have, with an electrical impulse. The result was, the heartbeat slowed down. Then he went one step further and took the blood substitute solution (physiological saline) from the ventricle with a pipette. He exchanged this with the solution in a second isolated frog heart, which then beat more slowly, just as if its vagus nerve had been irritated. Loewi had thus shown that the effect of vagus stimulation is mediated by a substance in the extracellular space. Only the solution from the pipette slowed down the heart rate of the second frog. This is the principle of chemical synaptic transmission and the transmitter substance is acetylcholine.
The transmitter, acetylcholine (ACh) is a neurotransmitter. What does that mean and where is it found?
Bornhorst: First of all, neurotransmitters are generally messenger substances of nerve cells. They are used to convert an electrical signal from a neuron into a chemical signal and can then cause an electrical signal in the next cell. This is the neurotransmitter. One of the most important neurotransmitters is acetylcholine, which is involved in many different processes. We find it in the central nervous system, i.e. in the brain and spinal cord, but also in the peripheral nervous system, i.e. outside the mentioned area.
This discovery was groundbreaking for the treatment of humans. Why?
Bornhorst: Everything we understand helps us find new therapeutic approaches. It is precisely our understanding of neurotransmitters in the brain that we use today in therapies for neurodegenerative diseases. In Alzheimer's disease, for example, there is a deficiency of acetylcholine, since acetylcholine-producing nerve cells also die off in the affected person. In Parkinson's disease, there is a relative excess of acetylcholine, which occurs when the demise of brain regions results in an imbalance between the neurotransmitters, i.e., an imbalance between acetylcholine and dopamine in favor of acetylcholine. Acetylcholine has a strong influence on motor function, and this is one of the reasons for the muscle tremor in Parkinson's disease. This knowledge has led to the discovery of therapeutic options.
The best-known transmitters include dopamine and serotonin, which have an excitatory as well as a depressant effect. How do you use these transmitters?
Bornhorst: Dopamine (DA) and serotonin (SRT) are monoamine neurotransmitters that play key roles in many aspects of mammalian nervous system function. The best-studied functions of dopamine in the nervous system include cognition, motor function, brain stimulation reward mechanisms, eating and drinking behavior, sexual behavior, neuroendocrine regulation, and selective attention. Changes in dopamine levels, that is, changes in its release in the body, lead to severe neurological and psychiatric disorders. The best known disorder is Parkinson's disease, which is caused by a loss of cells. Other disorders due to abnormal dopamine function include addiction, schizophrenia, bipolar disorder, Huntington's disease, attention deficit hyperactivity disorder, and also Tourette's syndrome.
Serotonin, in turn, has multiple effects in the human organism, particularly on the cardiovascular system, gastrointestinal tract, and nervous system. Serotonin is probably best known for its role in providing a sense of satisfaction and happiness. It is involved in virtually every type of behavior, such as appetitive, emotional, motor, cognitive, and autonomic functions. Alterations in the serotonergic system (SRTergic) play a role in many disorders, including depression, schizophrenia, migraine, anxiety, and dementia. This shows how important the correct regulation of neurotransmitters is for health.
What do you do when the neurotransmitters no longer function properly?
Bornhorst: Research is being conducted today on many neurodegenerative diseases. For example, we try to specifically compensate for a loss or deficiency by administering precursors of the neurotransmitters or by influencing synthesis/degradation. We should always have enough, but also not too much dopamine and serotonin in the body. The right balance is important. Now, if we have too little of something, it is always detrimental to health. In the case of Parkinson's disease, we therefore treat with levodopa (L-dopa), a precursor of dopamine. This is currently the most effective standard drug therapy, which, depending on the stage the patient is in, brings relief from the symptoms. Another option is so-called MAO and COMT inhibitors, which ensure that the dopamine is no longer broken down in the brain. These are just two approaches, although it must be said that to date we still have an inadequate understanding of neurodegenerative diseases. And for what we don't fully understand, we naturally lack suitable therapeutic approaches. There is a great need for research, especially to enable even better therapies, since the proportion of neurodegenerative diseases in the population is increasing.
To what extent do environmental factors and nutrition play a role?
Bornhorst: In Parkinson's disease, we know that only 10% is of genetic origin. So it is very important to identify substances in the environment that have a neurodegenerative potential. In our research, for example, we are looking at neurotoxicity from food/ and environmentally relevant metal compounds. Let me explain using manganese as an example: occupational or dietary overexposure to the trace element manganese can lead to toxic effects on the nervous system that can trigger a range of symptoms, such as gait changes, coordination disorders, hallucinations, or mental irritability. These eventually lead to an irreversible clinical picture known as manganism. This has a similar neuropathology to Parkinson` s disease with the presence of motor and cognitive impairments. The neurotoxic effects of manganese have been known for 175 years and its mechanisms of action have been intensively studied over the last century. Nevertheless, the mechanisms of manganese-mediated neurotoxicity are still poorly understood. L-dopa does not help there in the therapeutic approach, i.e., it must be something else mechanistic. A connection with oxidative stress, mitochondrial dysfunction, and protein aggregation is suspected but not yet proven. Age does not always explain everything; environmental factors also contribute to disease patterns. This is where I think we need to start. We need to find out to what extent environmental factors are a risk factor for neurodegenerative diseases and contribute to disease development. Then I can also try to get people to stop exposing themselves to certain risk factors. If certain metals are detrimental in an overdose, for example, we can pass that knowledge on to the population so that they have the opportunity to avoid it or protect themselves.
What models are you using at BUW for neurodegenerative diseases?
Bornhorst: We are looking at the influence of food- and environmentally relevant metal compounds on neuronal cells. There are different models. On the one hand cell culture models and on the other hand the nematode C. elegans.
In order to assess the neurotoxic or neuroprotective (positively influencing) efficacy of a compound transferred to the brain, we use human neurons in cell culture. For example, we were recently able to show that an oversupply of the trace element manganese has a negative effect on neurite growth - that is, the connections between the neurons. The network in the healthy organism (left), which is actually nice to look at, is destroyed by the disconnection of the connections (right). (Fig.1)
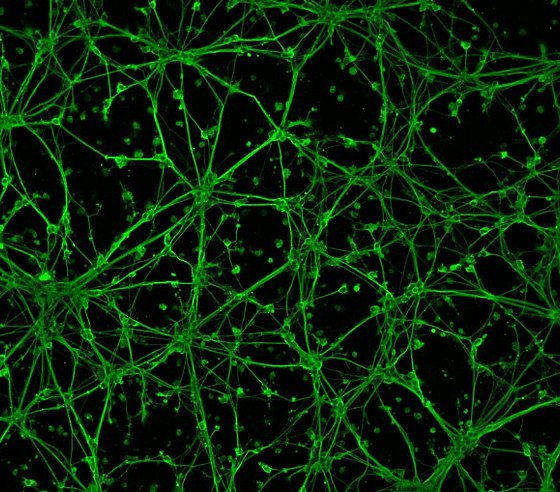
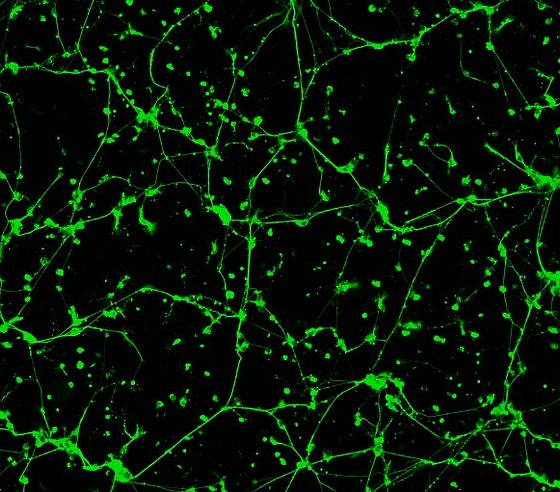
Fluorescence micrograph of human brain cells (neurons); the neuronal network can be seen in green. The left image shows untreated neurons, while the right image shows neurons after manganese oversupply (manganese is a trace element). Here, there is no longer an intact neuronal network. (Image: Food Chemistry Bergische Universität)
So we can see, these substances lead to neurodegeneration. And then we can investigate why this happens and possibly use substances so that this does not happen.
We also use the nematode C. elegans. This is the first multicellular organism whose genome has been completely sequenced, which makes it quite easy to genetically manipulate this model organism. Due to its highly specialized nervous system consisting of 302 neurons and more than 700 synapses, the worm also represents an innovative and powerful platform in terms of studying neuronal and possible neurodegenerative processes. Testing for possible neurotoxicity in the nematode can be done by different methods. One can use the transparent worms and specifically label their neurons with a fluorescent signal, i.e., stain them. In this way, one can microscopically identify appropriately affected neurons. When toxins are ingested, one can then see very clearly how the neuron has been damaged and is dying. Cell bodies are then only very small, connections are cut, so there is a neurodegenerative potential by a substance.
The behavior of the worm is also controlled by neurotransmitters. We can look at the looping rate, and if a worm is fed a metal and the looping rate changes, then we know immediately that something is wrong in the neurotransmitter system.
In addition, we are developing a method to also instrumentally-analytically detect the neurotransmitters, meaning we can quantify them, name the exact amount of dopamine, serotonin, or acetylcholine in worms, and make statements about how they are broken down. And we can also genetically manipulate the worm. We have so-called Parkinson's worms whose gene has been manipulated so that they have a high risk of Parkinson's disease. If we now want to know what the risk is for a neurodegenerative disease, so if the genetics have changed and we also feed the worm the metal, then we can see if it is more sensitive or less sensitive. So we can better understand and determine the genetics and the environmental factors.
Uwe Blass (conversation from 01.12.2021)
Julia Bornhorst studied and received her doctorate at the Westfälische Wilhelms University in Münster. She worked for five years at the Institute of Nutritional Science at the University of Potsdam. Since January 2019, she has been a professor of food chemistry at Bergische Universität.
